Uranium Enrichment
The following is an excerpt from the document Uranium Enrichment: Just Plain Facts to Fuel an Informed Debate on Nuclear Proliferation and Nuclear Power
Introduction to Uranium
There is one element that occurs in nature that has been the raw material for nuclear bombs: uranium, chemical symbol U. Uranium occurs in nature as a mixture of three different isotopes – that is, three different atomic weights that have virtually the same chemical properties, but different nuclear properties (see Appendix 1: Uranium: Its Uses and Hazards). These isotopes are U-234, U-235, and U-238. The first is a highly radioactive trace component found in natural uranium, but it is not useful in any applications; the second isotope is the only fissile material that occurs in nature in significant quantities, and the third is the most plentiful isotope (99.284 percent of the weight of a sample of natural uranium is U-238), but it is not fissile. U-238 can, however, be split by high energy neutrons, releasing large amounts of energy and is therefore often used to enhance the explosive power of thermonuclear, or hydrogen, bombs.
Because of the presence of small quantities of U-235, natural uranium can sustain a chain reaction under certain conditions, and therefore can be used as a fuel in certain kinds of reactors graphite-moderated reactors and heavy waterreactors, the latter being sold commercially by Canada). For the most common reactor type in use around the world today, which uses ordinary water as a coolant and moderator, the percentage of U-235 in the fuel must be higher than the 0.7 percent found in natural uranium. The set of industrial processes that are used to increase the percentage of U-235 in a given quantity of uranium go under the general rubric of “uranium enrichment” – with the term “enrichment” referring to the increase in the percentage of the fissile isotope U-235. Light water reactors typically use 3 to 5 percent enriched uranium – that is, the proportion of U-235 in the fuel is 3 to 5 percent, with almost all the rest being U-238. Material with this level of U-235 is called “low enriched uranium” or LEU.
Nuclear bombs cannot be made from natural or low enriched uranium. The proportion of U-235, which is the only one of the three isotopes that can sustain a chain reaction in uranium, is just too small to enable a growing “super-critical” chain reaction to be sustained. Uranium must have a minimum of 20 percent U-235 in it in order to be useful in making a nuclear bomb. However, a bomb made with uranium at this minimum level of enrichment would be too huge to deliver, requiring huge amounts of uranium and even larger amounts of conventional explosives in order to compress it into a supercritical mass. In practice, uranium containing at least 90 percent U-235 has been used to make nuclear weapons. Material with this level of enrichment is called highly enriched uranium or HEU. The bomb that destroyed Hiroshima on August 6, 1945, was made with approximately 60 kilograms of HEU. Highly enriched uranium is also used in research reactors and naval reactors, such as those that power aircraft carriers and submarines. The HEU fuel meant for research reactors is considered particularly vulnerable to diversion for use in nuclear weapons.
Gaseous Diffusion
As mentioned above, uranium is not able to be used as a weapon or as a fuel in its natural state. To be used as a fuel source, the uranium must be 'enriched' to have a higher percent concentration of U-235. To facilitate this enrichment, there are many processes that were developed during World War II and afterwards.
At the PGDP, the process used to enrich uranium is the gaseous diffusion process. Since in its natural state uranium contains a small concentration of the isotope U-235 (compared to over 99% U-238), the U-235 can be separated from the mixture by means of gaseous diffusion. The following figure shows a drawing of the process:
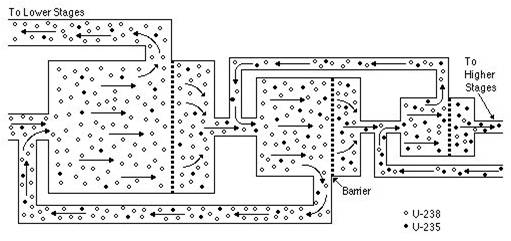
As the gas moves through the diffusion process, the lighter U-235 elements are separated from the heavier U-238 based on their differing velocities. The lighter U-235 elements move faster than the U-238 elements and this allows them to be separated. The process shown above must be repeated hundreds to thousands of times to achieve the desired enrichment level.
Further Reading
The following report outlines the history of enrichment technologies. It was produced in 2004 by the Institute for Energy and Environmental Research for the Nuclear Policy Research Institute.
The report outlines the basics of uranium enrichment, the science behind it, the current technologies, and examples of its use in the United States.
Plutonium
Plutonium is formed as a by-product of the decomposition of Uranium, as shown in the following diagram:
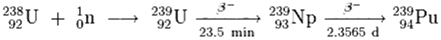
Neutrons from the fission of uranium-235 are captured by uranium-238 nuclei to form uranium-239; a beta decay converts a neutron into a proton to form Np-239 (half-life 2.36) and another beta decay forms plutonium-239. Plutonium-238, an isotope of plutonium-239, can be used as a source of energy. One kilogram of Pu-238 can generate about 570 watts of heat energy. For comparison, one kilogram of coal can produce about 2000 watts of heat energy, but also produces 3 kilograms of CO2.
The following table shows the Isotopes of Plutonium and specific information about the characteristics of each specimen.
| Isotope | Half-life (years) | Decay heat (W/kg) | Spontaneous fission neutrons (1/(g*s)) | Comment |
| Pu-238 | 87.7 | 560 | 2600 | Very high decay heat. Even in small amounts can cause significant self-heating. Used on its own in radioisotope thermoelectric generators. |
| Pu-239 | 24100 | 1.9 | 0.022 | The principal fissile isotope in use. |
| Pu-240 | 6560 | 6.8 | 910 | The principal impurity of the Pu-239 isotope. The plutonium grade is usually listed as percentage of Pu-240. High spontaneous fission hinders use in nuclear weapons. |
| Pu-241 | 14.4 | 4.2 | 0.049 | Decays to americum-241: its buildup presents a radiation hazard in older samples. |
| Pu-242 | 376000 | 0.1 | 1700 |
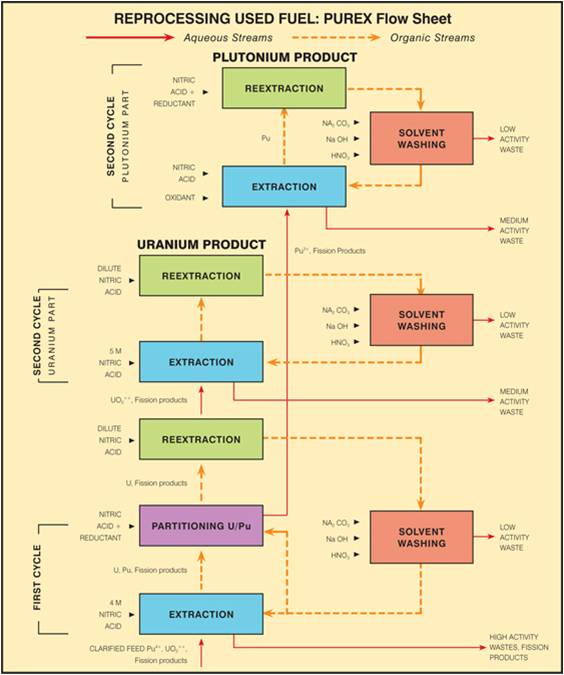
Once a nuclear fuel has been used in the production of electricity; it is possible to reclaim usable material from the spent nuclear fuel. This process is laid out in the following diagram: